Newly Arrival Test Antigen - KaiBiLi COVID-19 Antigen – Genesis
Newly Arrival Test Antigen - KaiBiLi COVID-19 Antigen – Genesis Detail:
Introduction
COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main symptoms including manifestations include fever, fatigue and dry cough, nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
The KaiBiLiTM COVID-19 Antigen Rapid Test Device is an in vitro diagnostic test based on the principle of immunochromatography for the qualitative detection of 2019 Novel Coronavirus nucleocapsid protein antigens in nasal swab or nasopharyngeal swab. The detection is based on the antibodies which were developed specifically recognizing and reacting with the nucleoprotein of 2019 Novel Coronavirus. It is intended to aid in the rapid diagnosis of SARS-CoV-2 infection.
This assay is intended for rapid screening in laboratory. This test should be conducted by trained technician, wearing appropriate personal protective equipment (PPE).
Detection
The qualitative detection of 2019 Novel Coronavirus nucleocapsid protein antigens in nasal swab or nasopharyngeal swab.
Specimen
Nasal or nasopharyngeal
Limit of Detection (LoD)
2019 Novel Coronavirus: 140 TCID50/mL
ACCURACY (Nasal swab)
Positive Percent Agreement: 96.6%
Negative Percent Agreement:100%
Overall Percent Agreement: 98.9%
ACCURACY (Nasopharyngeal swab)
Positive Percent Agreement: 97.0%
Negative Percent Agreement:98.3%
Overall Percent Agreement: 97.7%
Time to Results
Read results at 15minutes and no more than 30 minutes.
Kit storage conditions
2~30°C.
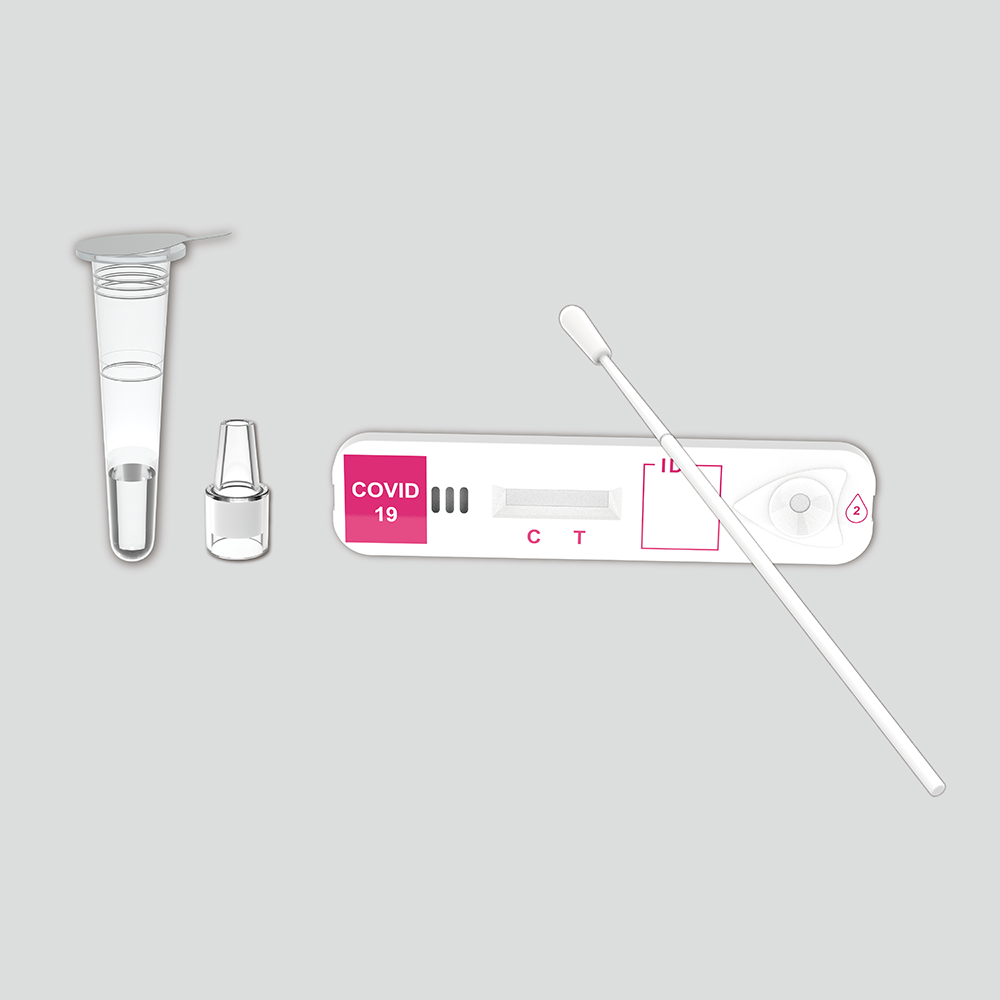
Contents
|
Description |
Qty |
| COVID-19 antigen test devices |
20 |
| Sterilized swabs |
20 |
| Extraction tubes (with 0.5mL extraction buffer) |
20 |
| Nozzles with filter |
20 |
| Tube Stand |
1 |
| Package Insert |
1 |
Ordering Information
|
Product |
Cat.No. |
Contents |
|
KaiBiLiTM COVID-19 Antigen |
P211139 |
20 Tests |
Product detail pictures:


Related Product Guide:
Bear "Customer first, Quality first" in mind, we work closely with our customers and provide them with efficient and professional services for Newly Arrival Test Antigen - KaiBiLi COVID-19 Antigen – Genesis , The product will supply to all over the world, such as: Montreal, Bhutan, Uganda, Our company always provides good quality and reasonable price for our customers. In our efforts, we already have many shops in Guangzhou and our products have won praise from customers worldwide. Our mission has always been simple: To delight our customers with best quality hair products and deliver on time. Welcome new and old customers to contact us for the future long term business relationships.
The company account manager has a wealth of industry knowledge and experience, he could provide appropriate program according our needs and speak English fluently.