Discountable price Antigen Test Detects - KaiBiLi COVID-19 IgG/IgM – Genesis
Discountable price Antigen Test Detects - KaiBiLi COVID-19 IgG/IgM – Genesis Detail:
Introduction
Any reactive specimen with the COVID-19 IgG/IgM Rapid Test Device must be confirmed with alternative testing method(s) and clinical findings. A positive test result requires further confirmation. A negative results do not preclude acute 2019-nCoV infection. If acute infection is suspected, direct testing for COVID-19 antigen is necessary. False positive results for COVID-19 IgG/IgM Rapid Test may occur due to cross-reactivity from preexisting antibodies or other possible causes. Due to the risk of false positive results, confirmation of positive results should be considered using second, different IgG or IgM assay.
Detection
The COVID-19 IgG/IgM Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative lateral flow immunechromatographic assay for the detection of IgG and IgM antibodies to 2019-nCoV in whole blood, serum or plasma specimen.
Specimen
Whole blood, Serum or Plasma specimen.
Accuracy
IgG Result:
Relative Sensitivity: 98.28%
Relative Specificity: 97.01%
Accuracy: 97.40%
IgM Result:
Relative Sensitivity: 82.76%
Relative Specificity: 98.51%
Accuracy: 93.75%
Time to Results
Read results at 15minutes and no more than 30 minutes.
Kit storage conditions
2~30°C.
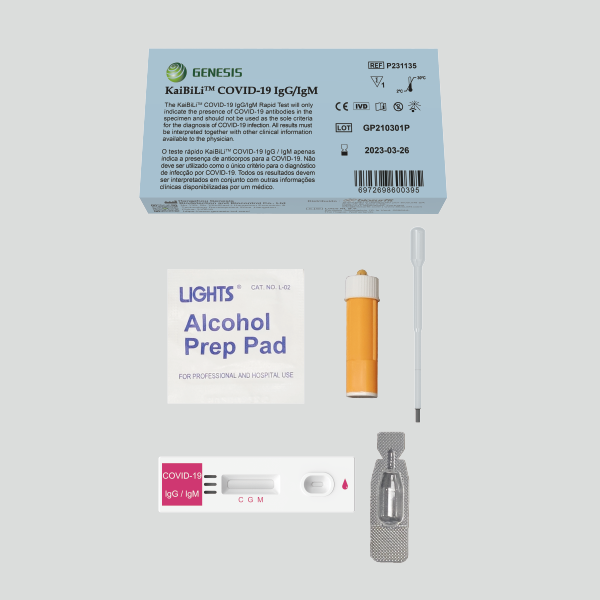
Contents
| P231133 | P231134 | P231135 | |
| COVID-19 IgG/IgM test device | 40 pcs | 30 pcs | 1 each |
| Sample buffer | 5mL/Bot.1Bot | 80μl/vial 30 vial | 80μl/vial 1 vial |
| Capillary dropper* | 40 pcs | 30 pcs | 1 each |
| Package insert | 1 each | 1 each | 1 each |
*Capillary dropper: For whole blood.
Ordering Information
|
Product |
Cat.No. |
Contents |
| KaiBiLiTM COVID-19 IgG/IgM | P231133 | 40 Tests |
| KaiBiLiTM COVID-19 IgG/IgM | P231134 | 30 Tests |
| KaiBiLiTM COVID-19 IgG/IgM | P231135 | 1 Tests |
Product detail pictures:

Related Product Guide:
Sticking to the principle of "Super High-quality, Satisfactory service" ,We are striving to generally be a very good business partner of you for Discountable price Antigen Test Detects - KaiBiLi COVID-19 IgG/IgM – Genesis , The product will supply to all over the world, such as: Belgium, Burundi, Paris, After years of development, we have formed strong ability in new product development and strict quality control system to ensure excellent quality and service. With the support of many long term cooperated customers, our products are welcomed all over the world.
The product manager is a very hot and professional person, we have a pleasant conversation, and finally we reached a consensus agreement.